Friday 5th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:

YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
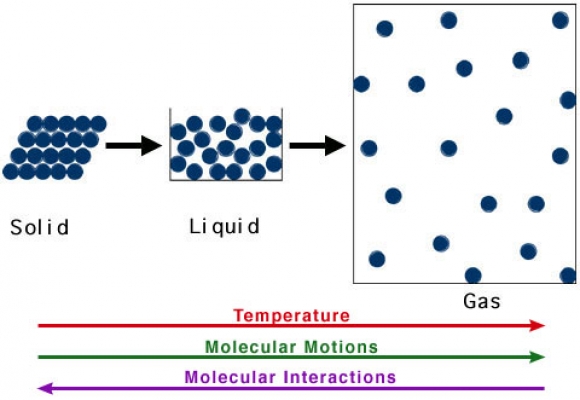
- Draw a labelled diagram to show the particle arrangement for a material sample as a solid, liquid and gas. Like:
- http://www.chemistry.wustl.edu/~edudev/LabTutorials/Thermochem/images/SolLiqGas.jpg
- Check for number of particles being constant.
- Label melting, freezing, evaporation, sublimation, condensation.
Super Challenge:
Stretch:
Challenge:
Key Questions:
- How does the mass of water change as an ice cube melts?
- The mass of water stays the same as an ice cube melts.
- What does the term 'conservation of mass' mean?
- Particles can not be created or destroyed.
- Why does the mass change during evaporation?
- Although there are no Particles destroyed during evaporation, once a gas the Particle can not be measured by the balance.
- How can we represent the changes of state in diagram form?
- Check for number of Particles being constant; Label melting, freezing, evaporation, sublimation, condensation.
