Friday 5th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:
YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
Super Challenge:
Stretch:
Challenge:
Key Questions:
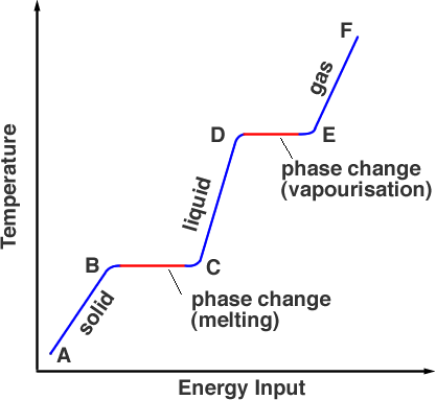
- What type of energy do particles gain when they are heated?
- Particles gain kinetic (movement) Energy when they are heated.
- How does the internal energy of a material change as state changes?
- A change of state from solid to liquid, liquid to gas or solid to gas requires an increase of the internal Energy of the material.
- Why does the temperature of water not increase during melting and evaporation?
- An increase of the internal Energy of the material does not always result in a increase in temperature. Some Energy is used in order to change state.
