Saturday 6th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:

YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
Super Challenge:
Stretch:
Challenge:
Key Questions:
- What is a mixture?
- A mixture consists of two or more elements or compounds not chemically combined together.
- What happens to the chemical properties of each substance in a mixture?
- In a mixture the chemical properties of each substance in the mixture are unchanged.
- When mixtures are separated by physical processes how does this affect the chemical reactions and substances made?
- Mixtures separated by physical processes do not involve chemical reactions and no new substances are made.
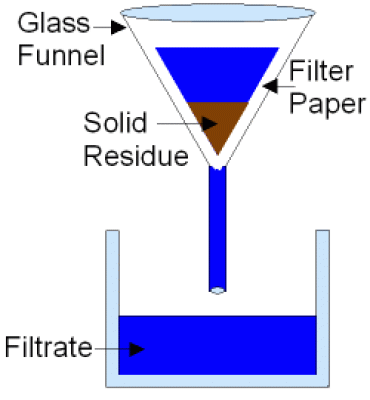
- How are mixtures separated using filtration?
- Mixtures of insoluble solid molcules and liquids can be seperated using filitration. The insoluble solid is unable to pass through filter paper allowing them to be separated from a liquid. For example a when a mixture of sand and water is filtered the sand remains in the filter and the water passes through.
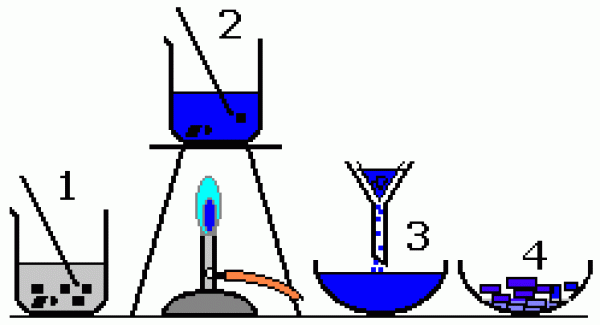
- How are mixtures separated using crystallisation?
- Crystallisation can be used to separate a solid that has dissolved in a liquid. The mixture is heated to remove most of the liquid and then left to allow crystals of the solid to form
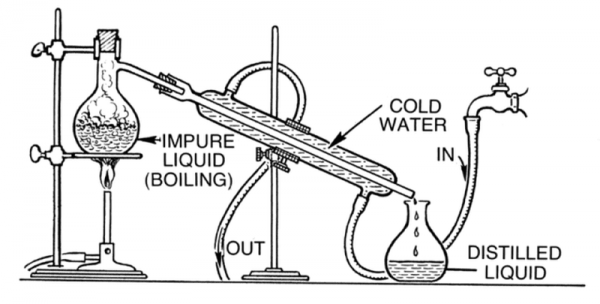
- How are mixtures separated using simple distillation?
- Distillation is a process that can be used to separate a pure liquid from a mixture of liquids. It works when the liquids have different boiling points. Distillation is commonly used to separate ethanol - the alcohol in alcoholic drinks - from water.
