Friday 5th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:

YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
Super Challenge:
Stretch:
Challenge:
Key Questions:
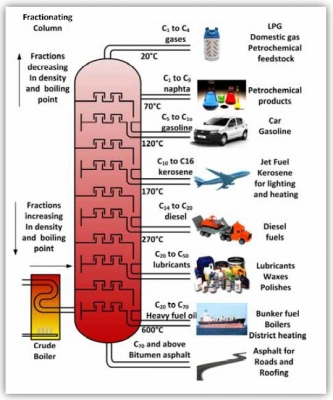
- How are mixtures separated using fractional distillation?
- During fractional distillation a mixture is separated into its component parts, or fractions, based on their boiling point. This is achieved by heating them to a temperature at which one or more fractions of the compound will vaporise and condensing them at different temperatures in a distillation column.
- How are mixtures separated using chromatography?
- In chromatography a mixture is passed through a medium in which the components move at different rates allowing separation of the individual components.
- How could we separare a mixture of sand, salt, iron and water into its individual components?
- A mixture of sand, salt, iron and water can be seperated using filtration to separate out the insoluble solids (iron and sand) from the solution (salt and water). Iron can be separated from the sand using a magnet. Salt and water can be separated by either evaporation or distillation.
