Saturday 6th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:
YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
Super Challenge:
Stretch:
Challenge:
Key Questions:
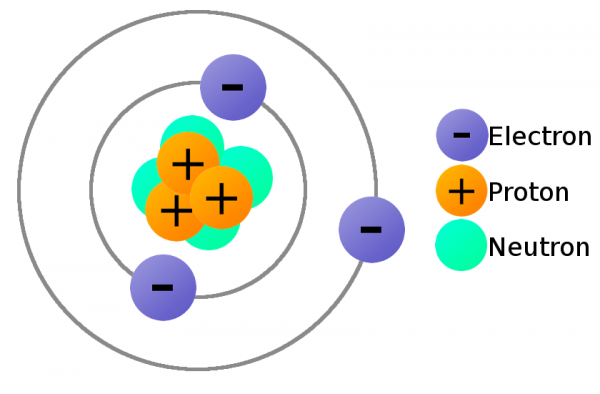
- What are the relative electrical charges of each sub-atomic particle?
- The relative electrical charges of each sub-atomic Particles are: Proton 1 Neutron 0 Electron -1
- Why do atoms have no overall charge?
- Atoms do not have an overall charge as a number of POSITIVE protons will always match the number of NEGATIVE electrons.
- If an atom has 9 protons, how many electrons would it have?
- If an atom has 9 protons it would have 9 electrons.
- What does the atomic number of an element tell us?
- The atomic number tells us the number of protons found in an atom of that element.
- What do all atoms of a particular element have in common?
- Atoms of the same element all have the same number of protons.
- What are the differences between an atom of Nitrogen and an atom of Carbon. What is it that makes them unique elements?
- An atom of carbon has: Protons= 6 Electrons= 6 Neutrons= 6. An atom of Nitrogen has: Protons= 7 Electrons= 7 Neutrons= 7. It is the differing number of protons which makes them unique elements.
- How does the current nuclear model describe an atom?
- The nuclear model shows that an atom is made of a small nucleus of positive protons and neutral neutrons, it also has negatively charged electrons orbiting it in Energy levels.
- How large is the radius of the nucleus of an atom in comparison to its total radius?
- The radius of the nucleus of an atom compared to the total atoms radius is 1/10 000 of that of the atom (about 1 x 10 to the power -14 m).
- Where is the majority of the mass of the atom found?
- The majority of the mass of the atom is found within the nucleus and is made up of protons and neutrons. Electrons have a negligible mass.
- What are the relative masses of each subatomic particle (proton, neutron and electron)?
- The relative masses of the subatomic Particles are: Proton = 1 Neutron = 1 Electron = negligible (very small)
- What does the mass number of an atom tell us about that atom?
- The mass number tells us the total number of protons and neutrons within that atom.
