Friday 5th September
TODAY WE ARE
LEARNING ABOUT
LEARNING ABOUT
TODAY'S
KEY WORDS ARE
KEY WORDS ARE

Memory Anchor:
YOU WILL SHOW
YOUR LEARNING BY...
YOUR LEARNING BY...
Super Challenge:
Stretch:
Challenge:
Key Questions:
- What is the radius of an atom?
- Atoms are very small, having a radius of about 1 x 10^-10 metres.
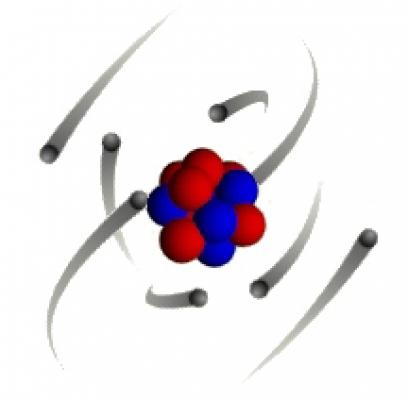
- What is the structure of an atom, with charges for sub-atomic particles?
- The basic structure of an atom is a positively charged nucleus composed of both protons and neutrons surrounded by negatively charged electrons.
- How does the radius of an atom compare with the radius of the nucleus of an atom?
- The radius of a nucleus is less than 1/10,000 of the radius of an atom.
- Where is most of the mass of an atom concentrated?
- Most of the mass of an atom is concentrated in the nucleus.
- How do energy levels differ in terms of distance from the nucleus?
- The electrons are arranged at different distances from the nucleus (different Energy levels).
- What might cause the electron arrangements to change (from a lower to higher energy level, for example)?
- The electron arrangements may change with the absorption of electromagnetic radiation (move further from the nucleus; a higher Energy level) or by the emission of electromagnetic radiation (move closer to the nucleus; a lower Energy level)
