
When choosing a radioisotope to use in an application we consider the type of radiation emitter and its half life. The type of radiation will be chosen based on its absorption properties, and the half life needs to be of an appropriate duration. Long half lives give stable activity. Short half lives reduce their acitivty to harmless amounts relatively quickly.
Smoke detectors consist of 2 main parts, an α emitter (usually Americium-241) and a radiation sensor. While the sensor is detecting radiation the smoke detector does not sound. When smoke gets in between the emitter and sensor then the alpha particles are absorbed by the smoke and no longer reach the sensor. The alarm then sounds.
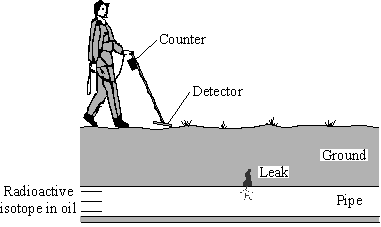 |
When a radioactive isotope is placed in a pipe's contents, then there will be a build up of radioactive activity around leaks.
Gamma rays can be used to 'x-ray' objects that an x-ray would not penetrate, such as metals. This is used in industry to check welds and such. |
See Gamma ray as part of electromagnetic spectrum
Radiation does most damage to fast growing cells. This allows it to be used to kill cancer cell since a tumour is just that. Side effects of chemotherapy is that the patients hair falls out, this is again due to them being rapidly growing cells.
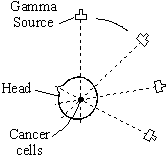
Gamma radiation can be used to kill cancer cells inside a personís body since it will penetrate the body. During the treatment the patient is kept perfectly still while the source of gamma radiation moves in a circle. This limits the damage done to health cells 'in line' with the tumour.
Have a go at treating a tumour
Doctors may use radioactive chemicals called tracers for medical imaging. Certain chemicals accumulate in different damaged or diseased parts of the body, and the radiation accumulates with it. Radiation detectors placed outside the body detect the radiation emitted and, with the aid of computers, build up an image of the inside of the body.
When a radioactive chemical is used this way it is important that:
Have a go at diagnosing a PET brain scan
Uranium-238 has a very long half-life. It decays via a series of short-lived radioisotopes to produce the stable isotope lead-204.
The relative proportions of uranium-238 and lead-204 in a sample of igneous rock can be used to date the rock. A rock sample contains three times as many lead atoms as uranium atoms.
This means that ___ / ___ of the original uranium is left in the rock, assuming that there was no lead in the original rock. This tell us that the rock has undergone ___ half lives. The half-life of uranium-238 is 4500 million years, so the rock is ___ years old.